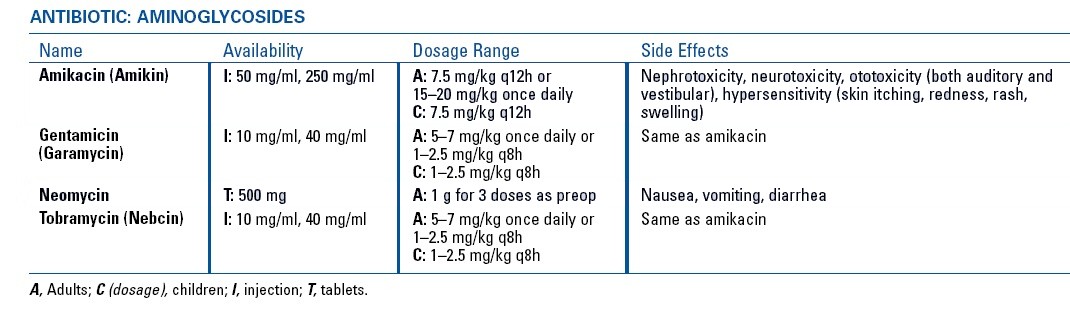
Aminoglycosides
Enumerate the common properites of aminoglycosides. Write the uses of gentamicin
Common properties
Not absorbed well thru GIT
Active against gram-negative organisms like Proteus, Klebsiella, Pseudomonas, Escherichia coli, Serratia and Enterobacter
Inactive against most gram-positive organismsm
Useful in the treatment of serious infections when other less-toxic agents are not effective or contraindicated, or require combination with penicillins or cephalosporins.
Used primarily for gram-negative microorganisms
Not well absorbed systemically from GI tract (must be administered parenterally for systemic infections).
Oral agents are given to suppress intestinal bacteria.
Action
Bactericidal.
Transported across bacteerial cell membrane;
Irreversibly bind to specific receptor proteins of bacterial ribosomes. Interfere with protein synthesis, preventing cell reproduction and eventually causing cell death.
Pharmacology
. Intravitreous injection is required to treat endophthalmitis. Intraventricular injection is often required to reach intraventricular levels high enough to treat meningitis.
Excreted by glomerular filtration and have a serum half-life of 2 to 3 h; the half-life increases exponentially as the GFR falls (eg, in renal insufficiency, in the elderly).
Indications
Acitive against facultative anaerobic bacilli
But lack activity against anaerobes and most gram-positive bacteria.
Active against most staphylococci; however, some gram-negative bacilli and methicillin-resistant staphylococci are resistant.
Preparations
Tobramycin
Gentamicin, and
Amikacin.
Streptomycin,
Neomycin
Kanamycin
. Amikacin is frequently active against gentamicin- and tobramycin-resistant pathogens.
For plague and tularemia.
They are usually used with a broad-spectrum ß-lactam for severe infection suspected to be due to a gram-negative bacillary species.
However, because of increasing aminoglycoside resistance, a fluoroquinolone can be substituted for the aminoglycoside in initial empiric regimens.
If the pathogen is found to be susceptible to the accompanying antibiotic, the aminoglycoside can be stopped after 2 to 3 days unless an aminoglycoside-sensitive P. aeruginosa is identified.
Gentamicin, streptomycin may be used with other antibiotics to treat endocarditis due to streptococci or enterococci. Enterococcal resistance to aminoglycosides has become a common problem. Because treatment of enterococcal endocarditis requires prolonged use of a potentially nephrotoxic and ototoxic aminoglycoside plus a bacterial cell wall–active drug (eg, penicillin, vancomycin) to achieve bactericidal synergy,
The choice of aminoglycoside must be based on special in vitro susceptibility testing. Susceptibility only to high levels of aminoglycosides in vitro predicts synergy when low-dose aminoglycoside therapy is combined with a cell wall–active drug. If the strain is susceptible to high levels of gentamicin and streptomycin, gentamicin is preferred because serum levels can be readily determined and toxicity is less. High-level enterococcal resistance to gentamicin in vitro does not rule out susceptibility of these strains to high levels of streptomycin; in such cases, streptomycin should be used.
Few therapeutic options are available for endocarditis due to enterococci that are resistant to high levels of gentamicin and streptomycin; no synergistic cell wall–active drug/aminoglycoside combination exists for endocarditis due to such strains, but the combination of the cell wall–active drugs ampicillin and ceftriaxone has recently been shown to be effective and minimizes the risk of nephrotoxicity.
Streptomycin has limited uses because of resistance and toxicity. It is used to treat tularemia and plague and, with other antibiotics, to treat TB.
Because of toxicity, neomycin and kanamycin are limited to topical use in small amounts.
Neomycin is available for eye, ear, oral, and rectal use and as a bladder irrigant.
Oral neomycin is used topically against intestinal flora to prepare the bowel before surgery and to treat hepatic coma.
Contraindications
AllergY to aminoglycosides.
Use During Pregnancy and Breastfeeding
Aminoglycosides are in pregnancy category D (there is evidence of human risk, but clinical benefits may outweigh risk).
Aminoglycosides enter breast milk but are not well absorbed orally. Thus, they are considered compatible with use during breastfeeding.
Adverse Effects
All aminoglycosides cause
Renal toxicity (often reversible)
Vestibular and auditory toxicity (often irreversible)
Prolongation of effects of neuromuscular blockers
Symptoms and signs of vestibular damage are vertigo and ataxia.
Risk factors for renal, vestibular, and auditory toxicity are
Frequent or very high doses
Very high blood levels of the drug
Long duration of therapy (particularly > 3 days)
Older age
A preexisting renal disorder
Coadministration of vancomycin, cyclosporine, or amphotericin B
For renal toxicity, coadministration of contrast agents
For auditory toxicity, a genetic predisposition, preexisting hearing problems, and coadministration of loop diuretics
High doses given over a long period of time typically cause more concern about renal toxicity, but even low doses given for a short time can worsen renal function.
Patients receiving aminoglycosides for > 2 wk and those at risk of vestibular and auditory toxicity should be monitored with serial audiography. At the first sign of toxicity, the drug should be stopped (if possible), or dosing should be adjusted.
Aminoglycosides can prolong the effect of neuromuscular blockers (eg, succinylcholine, curare-like drugs) and worsen weakness in disorders affecting neuromuscular transmission (eg, myasthenia gravis). These effects are particularly likely when the drug is given too rapidly or serum levels are excessively high. The effects sometimes resolve more rapidly if patients are given neostigmine or IV Ca. Other neurologic effects include paresthesias and peripheral neuropathy.
Hypersensitivity reactions are uncommon except for contact dermatitis due to topical neomycin. High oral doses of neomycin can cause malabsorption.
Dosing Considerations
Because toxicity depends more on duration of therapeutic levels than on peak levels and because efficacy is concentration-dependent rather than time-dependent (see Effectiveness), frequent doses are avoided. Once/day IV dosing is preferred for most indications except enterococcal endocarditis. IV aminoglycosides are given slowly (30 min for divided daily dosing or 30 to 45 min for once/day dosing).
In patients with normal renal function, once/day dosing of gentamicin or tobramycin is 5 mg/kg (7 mg/kg if patients are critically ill) q 24 h, and once/day dosing for amikacin is 15 mg/kg q 24 h. If patients respond to the 7-mg/kg dose of gentamicin clinically and renal function continues to be normal, the once/day dose can be reduced to 5 mg/kg after the first few days of treatment.
In critically ill patients, peak serum levels should be determined after the first dose. In all patients, peak and trough levels are measured after the 2nd or 3rd dose (when the daily dose is divided) or when therapy lasts > 3 days, as well as after the dose is changed. Serum creatinine is measured every 2 to 3 days, and if it is stable, serum aminoglycoside levels need not be measured again. Peak concentration is the level 60 min after an IM injection or 30 min after the end of a 30-min IV infusion. Trough levels are measured during the 30 min before the next dose.
Peak levels in serum of at least 10 times the MIC are desirable. Dosing is adjusted to ensure a therapeutic peak serum level (to facilitate concentration-dependent activity) and nontoxic trough levels (see Table: Dosing for Aminoglycosides in Adults). In critically ill patients, who are likely to have expanded volumes of distribution and who are given higher initial doses, target peak serum levels are 16 to 24 µg/mL for gentamicin and tobramycin and 56 to 64 µg/mL for amikacin. For gentamicin and tobramycin, trough levels should be < 1 µg/mL at 18 to 24 h after the first dose with once/day dosing and between 1 and 2 µg/mL with divided daily dosing.
For patients with renal insufficiency, the loading dose is the same as that for patients with normal renal function; usually, the dosing interval is increased rather than the dose decreased. Guidelines for maintenance doses based on serum creatinine or creatinine clearance values are available (see Table: Dosing for Aminoglycosides in Adults), but they are not precise, and measurement of blood levels is preferred.
If patients are taking a high dose of a ß-lactam (eg, piperacillin, ticarcillin) and an aminoglycoside, the high serum levels of the ß-lactam can inactivate the aminoglycoside in vitro in serum specimens obtained to determine drug levels unless the specimen is assayed immediately or frozen. If patients with renal failure are concurrently taking an aminoglycoside and a high-dose ß-lactam, the serum aminoglycoside level may be lower because interaction in vivo is prolonged.
Dosing for Aminoglycosides in Adults
1. Choose loading dose in mg/kg for peak serum levels in the range listed below for the aminoglycoside being used. If the patient’s actual weight is > 20% higher than ideal weight* because of obesity, the weight used for dosing equals ideal weight plus 40% of excess body weight (actual weight minus ideal weight). If actual weight exceeds ideal weight because of ascites or edema, the weight used for dosing is the actual weight.
Aminoglycoside
Usual Loading Doses
Gentamicin
Tobramycin
1.5–2.0 mg/kg
Amikacin
5.0–7.5 mg/kg